KUALA LUMPUR, July 15 — The National Pharmaceutical Regulatory Agency (NPRA) will deliver its decision on giving Sinopharm conditional approval to be used in the National Covid-19 Immunisation Programme tomorrow, Tan Sri Dr Noor Hisham Abdullah has revealed.
The Health director-general, during a press conference this evening, said the decision will come after almost a month’s long evaluation process of the Sinopharm vaccine dossiers by the NPRA.
“Tomorrow the NPRA will meet and we will consider the Sinopharm vaccine, which was approved by the World Health Organisation.
“Their company just sent their dossiers to us, and in less than a month we have evaluated and will give our decision on the conditional approval tomorrow,” he said.
With Sinopharm’s inclusion, NIP would have a total of six types of vaccines within their portfolio, adding to the list that includes the Pfizer, AstraZeneca, Sinovac, Cansino, and Johnson & Johnson vaccines.
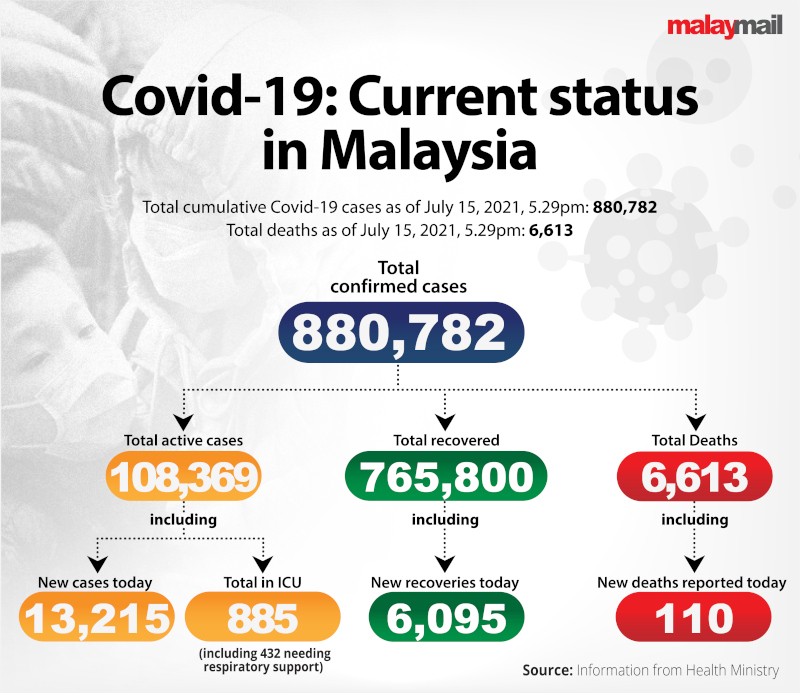
As of midnight today, a total of 8.6 million individuals have completed their first dose of Covid-19 vaccine jabs, with four million more having completed both doses.
Vaccination rates nationwide have hovered over 400,000 doses administered per day over the past few days, surpassing projections initially aimed at achieving 300,000 doses per day.